On September 27th, 2023, the National Agency for Pharmaceutical Products took part in the ‘VIII All-Russia GMP Conference’, under the theme ‘GMP: Good Quality in Present Context’, organized by the Ministry of Industry and Trade and the State Institute of Drugs and Good Practices (SID&GP) of the Russian Federation.
The aim of the conference was to bring together experts and government authorities from different countries, in order to harmonize their efforts and develop common approaches aimed at better regulation of good manufacturing practice and the drug market, to guarantee their quality, efficacy and safety.
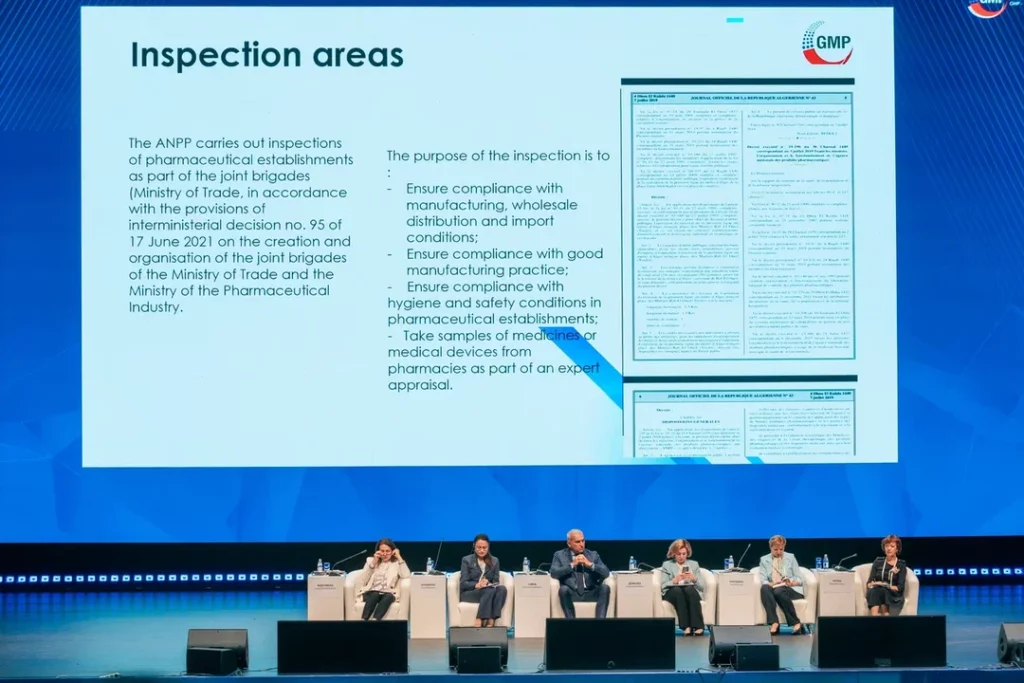
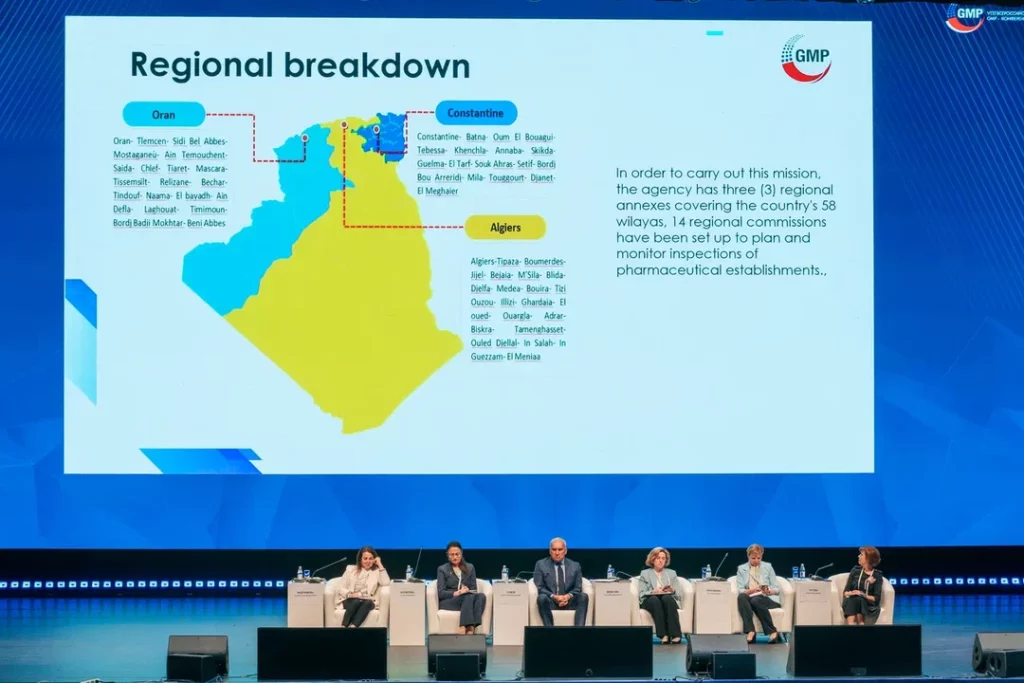
During its contribution to the panel session entitled ‘inter-agency cooperation for enhancing the pharmaceutical market’, ANPP presented a paper on the legislative and regulatory aspects of inspections of pharmaceutical manufacturing entities in Algeria.